Hepatocellular Carcinoma
(78) Evaluating the Safety and Efficacy Y-90 Radioembolization in Combination with Immunotherapy for Hepatocellular Carcinoma
Saturday, October 18, 2025
6:00 PM - 7:30 PM East Coast USA Time
Mina Makary, M.D. – Physician, Division of Vascular and Interventional Radiology, Department of Radiology, The Ohio State University Wexner Medical Center; Fady Bassem Fayek, B.S. – Medical Student, Sidney Kimmel Medical College; Emily Hashem, B.S. – Medical Student, Sidney Kimmel Medical College
Purpose: Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer and the third leading cause of cancer-related mortality globally. Yttrium-90 transarterial radioembolization (Y-90 TARE) is a catheter-directed locoregional therapy that directly induces cytotoxic injury to cancer cells while minimizing damage to healthy tissue. This educational exhibit discusses latest advances in the use of Y-90 TARE in combination with immune checkpoint inhibitors (ICI).
Material and Methods: A review of the latest clinical trials and studies of Y-90 TARE/ICI combination treatment is conducted to characterize the safety and efficacy of this emerging treatment for HCC. Findings are presented in text and figure format, identifying limitations and future directions of this research.
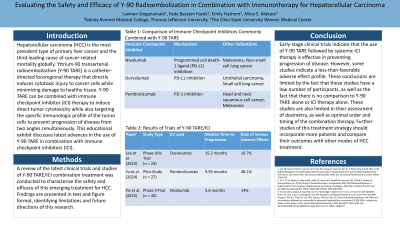
Results: The goal of combination Y-90 TARE/ICI therapy is to induce direct tumor cytotoxicity while also targeting the specific immunologic profile of the tumor cells to prevent progression of disease from two angles simultaneously. Several trials and studies have been done evaluating Y-90 TARE in combination with different ICIs including nivolumab, ipilimumab, durvalumab, and pembrolizumab. These retrospective studies and early-stage clinical trials indicate that the use of Y-90 TARE followed by systemic ICI therapy is effective in preventing progression of disease. They also found that this treatment modality has an acceptable safety profile, with little to no evidence of early secondary mortality or toxicity. These conclusions are limited by the fact that these studies have a low number of participants, as well as the fact that there is no comparison to Y-90 TARE alone or ICI therapy alone. These studies are also limited in their assessment of optimal order and timing of the combination therapy. Further studies of this treatment strategy should incorporate more patients and compare their outcomes with other modes of HCC treatment.
Conclusions: Combination Y-90 TARE/ICI is a therapy that is promising in its efficacy in preventing HCC progression as well as its relatively benign safety profile. However, further research with more patients into the order and timing of these therapies is needed before it can be wholeheartedly endorsed.
Material and Methods: A review of the latest clinical trials and studies of Y-90 TARE/ICI combination treatment is conducted to characterize the safety and efficacy of this emerging treatment for HCC. Findings are presented in text and figure format, identifying limitations and future directions of this research.
Results: The goal of combination Y-90 TARE/ICI therapy is to induce direct tumor cytotoxicity while also targeting the specific immunologic profile of the tumor cells to prevent progression of disease from two angles simultaneously. Several trials and studies have been done evaluating Y-90 TARE in combination with different ICIs including nivolumab, ipilimumab, durvalumab, and pembrolizumab. These retrospective studies and early-stage clinical trials indicate that the use of Y-90 TARE followed by systemic ICI therapy is effective in preventing progression of disease. They also found that this treatment modality has an acceptable safety profile, with little to no evidence of early secondary mortality or toxicity. These conclusions are limited by the fact that these studies have a low number of participants, as well as the fact that there is no comparison to Y-90 TARE alone or ICI therapy alone. These studies are also limited in their assessment of optimal order and timing of the combination therapy. Further studies of this treatment strategy should incorporate more patients and compare their outcomes with other modes of HCC treatment.
Conclusions: Combination Y-90 TARE/ICI is a therapy that is promising in its efficacy in preventing HCC progression as well as its relatively benign safety profile. However, further research with more patients into the order and timing of these therapies is needed before it can be wholeheartedly endorsed.
